Atomic Structure
Someone told me whilst I was training to teach that the concept of an atom is such an abstract thing that some people will just never understand it. I strongly beg to differ, the concept itself is incredibly simple to understand, it just requires some basic math to be applied.
Key Terms
proton, neutron, electron, orbital, shell, shielding, configuration, nucleus, attraction, repulsion, charge, mass, positive, negative, neutral, negligable, relative, element, atomic number, atomic mass.
Explanation
So all you really need to know about atomic structure is the following table:
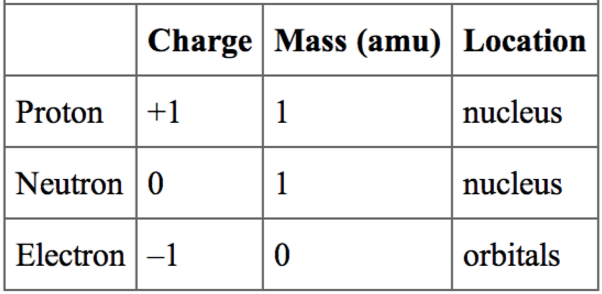
Literally that is all, it's not hard to remember, but in my experience students either tend to struggle for one of two reasons, usually because they are too lazy to learn 15 boxes but occasionally because they struggle to apply this, and that normally comes down to their mathematical skill.
Let's start off with the basics, the nucleus. Famously discovered by a man named Ernest Rutherford in an experiment with gold particles. He fired alpha radiation at a gold sheet and recorded where the alpha particles went, most went straight few, very occasionally they veered and even more rarely than that they bounced back. This showed the presence of protons.
This experiment showed that the size of the nucleus was a tiny fraction of the size of the atom, also meaning that an atom is mostly made of nothing, meaning you are mostly made of nothing (scary huh?)
The neutrons were the last to be discovered (mostly because they have no charge and are therefore difficult to identify). They have a few roles in the atom and realistically I am about to lie to you but in GCSE chemistry we refer to them as the glue of the nucleus. If you think about it a ball of positively charged particles are going to repel each other and break apart, the neutrons stop them from doing this, that's all.
Now that isn't too difficult is it? But why is this important I hear some of you thinking, well...
If you look at the table above you will notice that the only particles with any significant relative mass are protons and neutrons. Now look at your periodic table, each element has two numbers. The smaller number (it's atomic number) is the number of protons, this makes the element what it is, the defining characteristic of an element is it's number of protons. The bigger number is the element's atomic mass and this is the number of protons plus the number of neutrons.
Now we use the term relative for a very important reason here and it is important you understand why.
Protons and neutrons are tiny, so tiny in fact that their mass is so small it is very difficult to quantify (1/6.02x10^23g to be precise) so we refer to them in relative numbers that have huge significance later on in chemistry.
So we've got the nucleus, essentially the number of protons define the element and the neutrons hold the positive protons together, great!
Now electrons...
We've known about electrons longer than the others, right back to J.J.Thomson's plum pudding model (look it up!). They have a negative charge, that's important as we have protons in the nucleus with a positive charge and almost no mass (lots of acceptable answers to this question; 0, negligible, 1/2000, 1/1840 to name a few).
Now the mass has very little to do with the structure of the atom, but the charge however does. for each positive charge from the proton there is an equal negative charge from an electron. Therefore if you look at hydrogen that always has 1 proton, in an atom (not an ion) it has 1 electron. Potassium has 19 protons, so has 19 electrons. An atom always has a balanced charge so must have the same number of protons as electrons (again I will stress...not the same in ions, they are different to atoms).
The problem is however that electrons do not have neutrons to hold them together, they just have protons to attract them to the nucleus, so naturally they repel and therefore form orbitals that look like this:
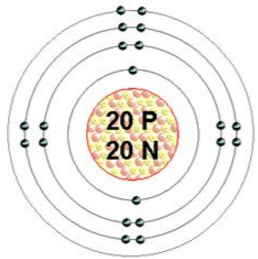
And now everyone panics as it isn't the pretty 3D structure at the start of Big Bang Theory. well there is no need, it's really simple.
Each black dot (normally represented as a dot or a cross) is an electron, and the first shell, the one closest to the nucleus is so small it can only hold 2 before the electrons are too close and repel each other away. However this is where the strongest attraction to the nucleus is so this is where the first electrons will go.
After that they go in to the second shell which can hold 8 before it gets to crowded, the next shell contains 8 again and the shell after that (although at GCSE you will never be tested on anything past the first two in calcium above) can hold 18.
The more protons an elements nucleus has the more electrons the atom can hold. The problem is the further away from the nucleus the electron is, the weaker the pull from the nucleus is, which is why it is only possible to get 7 electron shells (and only in very extreme conditions).
There is also something called electron shielding that adds to this effect, you see as there are more shells there are also more electrons between the positive nucleus and the negative outermost electrons, the increasing negative charge from the inner shells as the atom gets bigger further repels the outer most electron.
I will go into electron configuration in another chapter but in terms of atomic structure that is all you need to know.
Exams
This is usually a starter questions and often leads on to other stuff, usually they will give you the element and ask for the number of protons, neutrons and electrons in the atom, just remember the number of protons is the smaller number, then to get the number of neutrons take the small number from the big number. then the number of electrons is the same as the number of protons unless the atom has a charge in which case it is an ion not an atom.
They may also get you to draw it, I will do a whole chapter on electron configuration so don't worry about that until you have read that chapter.
Mistakes.
First and foremost students make mistakes because they do not know the table at the start of the chapter. The concept in this are basic addition really and should therefore be easy marks.
The other issue students have are their roles in the atom, be careful! Protons are responsible for the element, neutrons responsible for holding the protons together and the electrons are responsible for the chemical properties (this will be more obvious in other related chapters).
Tricks
I always remember it like this; I start with the Proton, always looking first on the bright side of life and keeping Positive, guess what they are positive. the Nucleus therefore contains Neutrons and so electrons must be negative and on the outside.
When it comes to atomic number and mass these are often got the wrong way around, most teachers tell you the bottom number is the atomic number, personally I find this very irresponsible as I have seen periodic tables with the atomic number at the top and bottom. The smaller number is always the atomic number as this is protons alone, the bigger is therefore the atomic mass as this is the number of protons and neutrons so it must be bigger.
Extension
There are too many to go into here, this really is the foundation of most chemistry at this level and above so most questions will start on this topic and then diverge into bonding, electronic configuration, reactivity etc.
Bạn đang đọc truyện trên: Truyen247.Pro