Water Stuff
Water Stuff
The German word for Hydrogen is Wasserstoff!
Water covers 71% of the Earth's surface and it is the most important chemical in the universe because all carbon based life needs it to not only form but to survive. And yet, what do we really know about water?
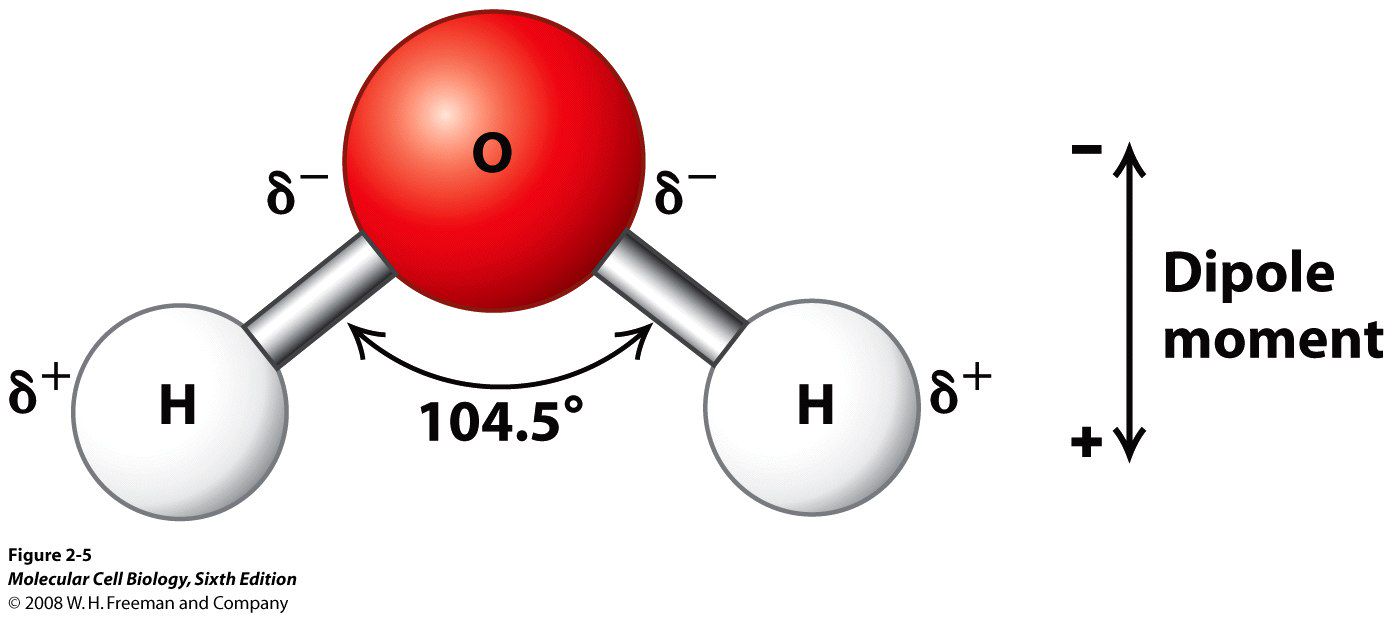
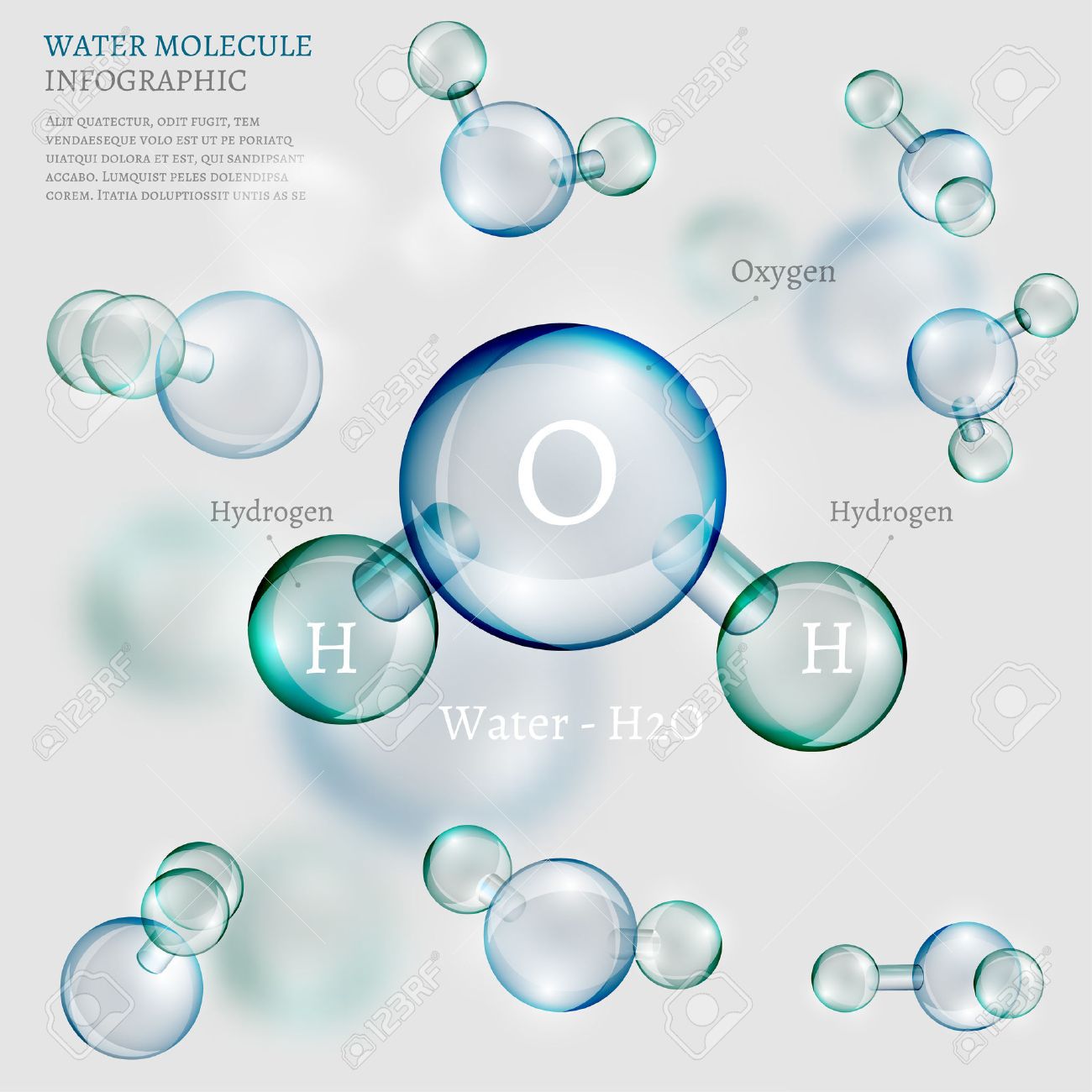
Almost everyone knows that water is H2O, which indicates that the water molecule is made up of 2 Hydrogen atoms and 1 Oxygen atom. The hydrogen atoms are bonded to the oxygen atom at an angle of 104.48 degrees, and this molecular arrangement is very important in explaining how water behaves in both a physical sense as well as a chemical sense. It's also the reason that water is considered the universal solvent.
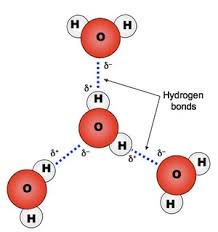
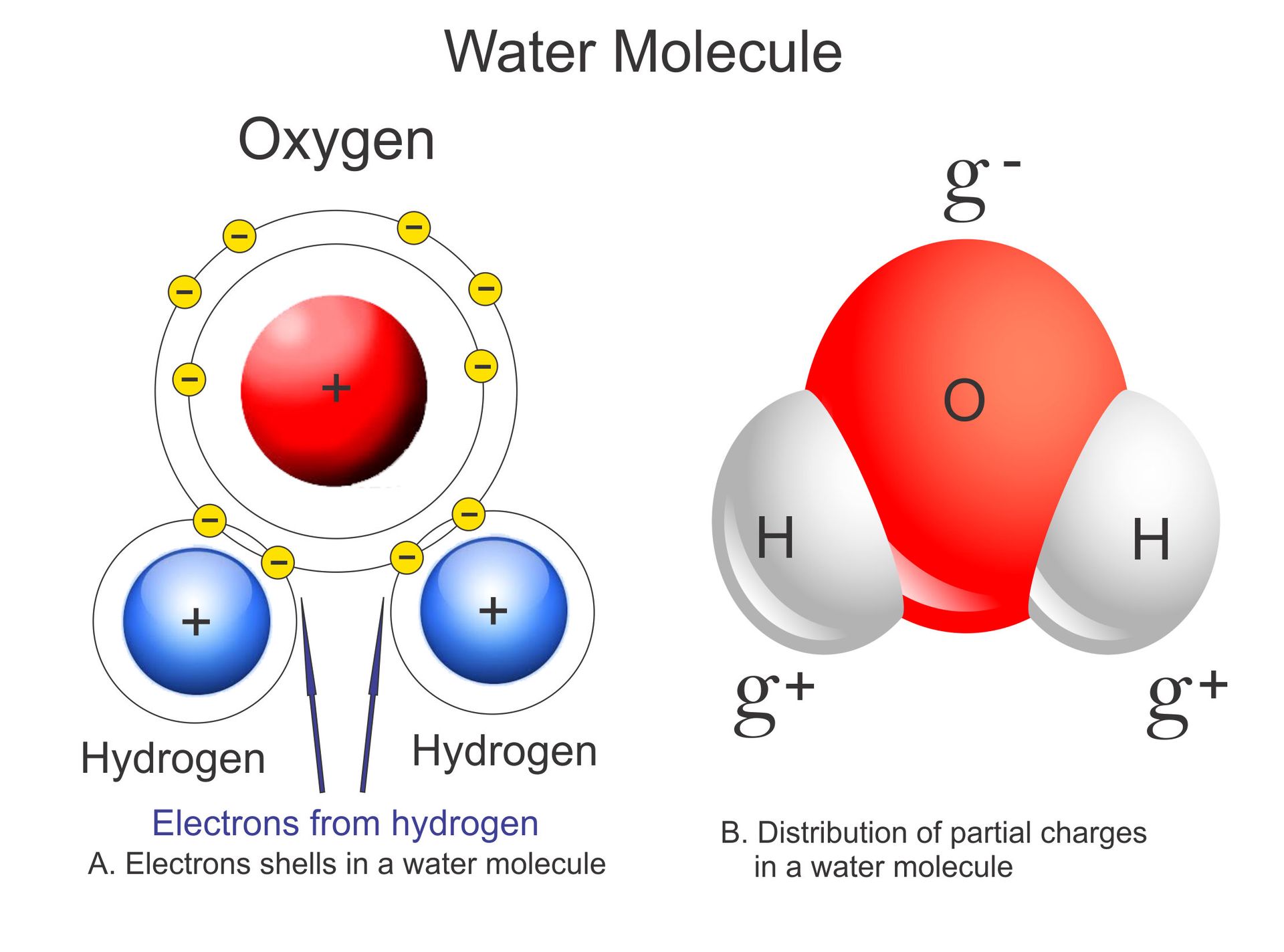
The molecular structure of water makes it polar. As you might know, atoms form bonds by sharing the electrons that orbit their nuclei. In the case of water, the electrons spend more time around the oxygen atom instead of the hydrogen atoms. This makes the water molecule polar and that means that water molecules can attract one another. They do this by what is called hydrogen bonding, a force that allows water to exist as a liquid at temperatures up to 100 C, which is the boiling point of water at sea level.
Hydrogen bonding is responsible for water molecules having phases; liquid, solid and gas. The changes of water among these three phases are determined by temperature and pressure. Some of these phases have numerous forms. For example, solid water can exist in several crystalline and amorphous forms. Consider the many strange structures of snowflakes as compared to ice cubes. There are 18 forms of ice.

One of the most important properties of water is that it has a lower density when it's frozen into ice than it does as a liquid. This means that ice floats on water, a property that permitted sea life to form and thrive. If ice were denser than liquid water, lakes and ponds would freeze from the bottom up and kill life. Water has a density of 1 gram per cubic centimeter near the freezing point of 0 C. Right below 0 C, water as ice has a density of 0.92 g/cm3. At the boiling point of 100 C, water has as density of 0.96 g/cm3.

Another very important property of water is capillary action, which in turn is related to surface tension. This is the property that allows plants to suck water up their stems against gravity.

The other most important property of water is its ability to dissolve many other chemicals. It does this because of its polarity. Most ionic compounds like salt, acids and bases easily dissolve in water. Organic chemicals dissolve if they can overcome the attractive forces between the molecules of water. However, it is this universal solvent property that allowed life to form and thrive. That's the reason that astrophysicists say that where there is water, there could be life.
The fact is that water has all of the physical and chemical properties to foster life. Without it we bags of water would not exist. We are essentially water stuff.
Despite this, we do not know everything about water. It's still a scientific curiosity.
Thanks for reading.
Bạn đang đọc truyện trên: Truyen247.Pro