Quantum Physics
Quantum Physics
We all have heard of this branch of physics. Quantum particles make up the atoms that we see and the electromagnetic energy that we feel and observe. But, how did they determine that quantum particles actually exist?
To start with, this branch of physics was called quantum mechanics because of the way that they described the motions of particles. This concept began back in the 19th century when Tomas Young did the 'Double-slit' experiment. Physicists had suspected that electromagnetic energy such as visible light displayed both wave and particle attributes. That's where the idea of quantum comes from. Light can behave as discrete packets of energy, which were called quanta, and it can also behave like a wave, which has frequency and wavelength. The different colors that we see are based on the differing frequencies of light energy, acting like waves.
This is where the double slit experiment comes into play. If one passes coherent light-such as from a laser-through two parallel close slits, the light shining on a screen past the slits shows dark and light bands. Interference effects that are attributed to the wave nature of light cause these bands. But, at the same time the light forms discreet bands that are attributed to the particle nature of light. This experiment is where the wave-particle duality of light came from.
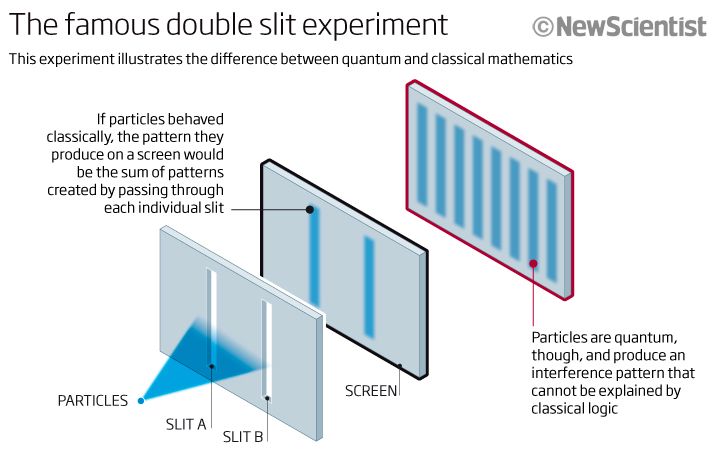
One could argue that the effect is either one or the other cause, but if the experiment is done with an electron beam, the same result is obtained. In other words a particle, such as the electron, behaves both as a wave and a particle.
Think of it this way. If you fire a beam of light or a beam of electrons through one slit, you end up with a diffraction pattern at the exit side in which the light or the electrons are spread out. The smaller the slit, the more spread out the result is. This is the result of diffraction, which is a wave property.
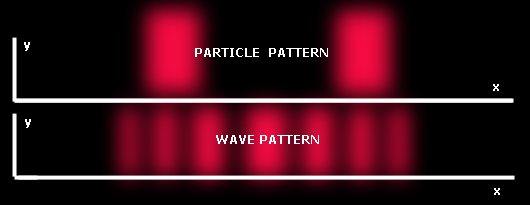
If you shoot the beams through two slits close together, you end up with a series of light and dark bands, which can only be attributed to the discreet particle nature of light. The conclusion is that a single particle can be in two places at the same time. This is a basic fact of quantum physics.
If you position detectors at the slits, the interference pattern disappears. This illustrates another basic fact of quantum physics. If you try to observe or measure the particles you will either see them as waves or as particles but not both. In essence, this is the idea that a measurement affects a quantum result. Those pesky particles are sneaky.
This is how quantum physics originated. It was soon obvious that tiny quantum particles behave differently than macro objects. Eventually, with the use of particle accelerators, physicists found many other particles, such as the quarks that make up baryonic matter such as atoms. What this demonstrated is that quantum particles can only be described using probability. There is no way to know the precise location, spin and energy of any particle at the same time.
Quantum particle pairs can be spin-synced even at a great distance, and these particles can pop in and out of existence. Welcome to the wacky world of quantum physics, which started with the double-slit experiment.
Thanks for reading.
Bạn đang đọc truyện trên: Truyen247.Pro