Combustion
Combustion
This subject is very important to the understanding of fire safety. As you probably know, combustion is when something burns in air. In other words it's the reaction of a combustible material with oxygen.
Fire is probably one of the first important discoveries of early humans. Of course, they had no idea what was going on, but it didn't matter. Fire provided light and heat. Early humans found that heat can cook food, like meat, and make it less dangerous to eat. Without fire, it's doubtful if early humans would have survived.
Combustion was also important in the establishment of the industrial revolution. Coal was used to power steam engines to move trains and provide power for industries. Basically, organic compounds, carbon, and hydrogen are the most used materials to provide heat and power by means of combustion.
What happens during combustion? Oxygen reacts with the combustible material to provide heat and light. That's why combustible materials are also called combustibles. Organic compounds that are used for combustion include, methane, ethane, butane, propane, ethanol, methanol and petroleum distillates such as kerosene, gasoline and fuel oil or diesel.
In the case of hydrocarbons contained in gasoline, the reaction involves oxygen combing with carbon to make carbon dioxide and carbon monoxide. Hydrogen atoms get converted to water. If the fuel contains sulfur, one obtains sulfur dioxide.
The temperature of combustion varies with the combustible used and the air to fuel ratio. Some fuels react better if mixed with air first. This is very important in the case of gasoline. If one takes ethanol and pours it into a large water bottle and swirls it around to make it vaporize slightly, a spark will cause it to burn with a fairly bright yellow flame if it's not pure ethanol. Pure ethanol burns faster and the flame is almost invisible. In this same experiment gasoline explodes, indicating that it burns very rapidly. It also melts the bottle. The ethanol doesn't.
Hydrogen has the highest heat of combustion at 141.8 MJ/kg - mega joule per kilogram. Propane is 50.35 MJ/kg. Diesel is 44.80. Coal is 15 to 32.5, depending if it's ignite or anthracite, which is the best. Wood is only 21.7. Gasoline is roughly in the middle at around 48 - 50.
Air-fuel ratio is very important to understanding how flammable combustibles are. It's also important for combustion engines. Gasoline has an air to fuel ration of 14.7 to 1. Hydrogen is 34.3 to 1, while Ethanol is only 9 to 1. At these ratios, the fuel is burned completely.
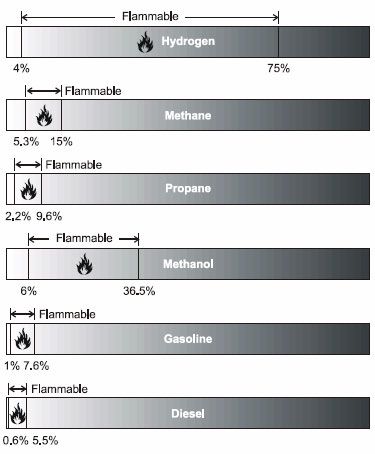
The other very important parameter is flammability limits. These are the limits of fuel to air by volume in which the fuel will burn or explode. These are based on the fuel being in a vapor form. With most combustibles this will be the case because of evaporation.
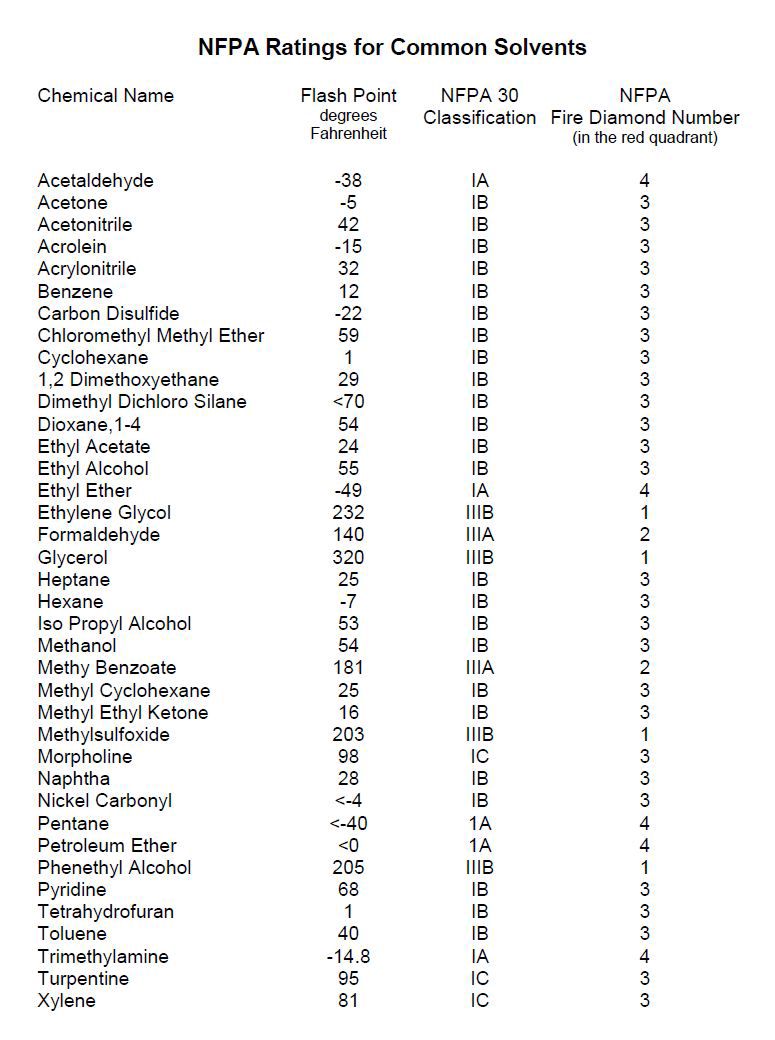
Another parameter is the flash point. This is the temperature at which the fuel if at proper mixture with air will explode if exceeded. For example Ethanol has a lower limit of 3.3 and upper limit of 19, with a flash point of 12.8 C. This means that as long as ethanol is not above 55 F it won't burn.
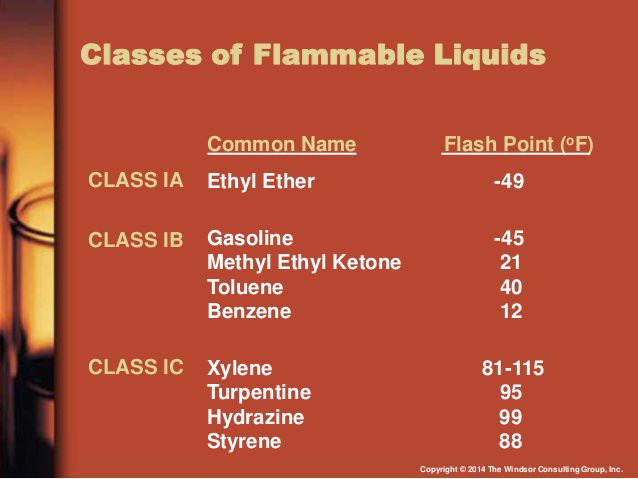
Gasoline (100 octane) is 1.4 for lower limit, 7.6 of the upper limit. This is not a very large range. The flash point is -40 C. That's very flammable.
Diethyl Ether has a lower limit of 2 and upper limit of 36-48 with flash point of -45 C. Believe me this is extremely flammable. You don't even need a spark, just something hot to make it ignite.

The reason why I am posting this is because I feel that a lot of people don't realize how dangerous gasoline is. The best way to store gasoline is in a solvent safety can. These are made out of steel and have a brass flash barrier in the nozzle. These cans can be thrown on a fire filled with gasoline and they will never explode. They will vent out the gasoline and the vented fuel will burn but the can will not explode because the flash barrier prevents a flame or spark from entering the can. I know this is true because I've seen it demonstrated.
Thanks for reading.
Bạn đang đọc truyện trên: Truyen247.Pro